| TRIMETHYLENE GLYCOL | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 504-63-2 |
|
| EINECS NO. | 207-997-3 | |
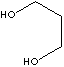
| FORMULA | HO(CH2)3OH | |
| MOL WT. | 76.10 | |
|
H.S. CODE |
||
| TOXICITY | ||
| SYNONYMS | Beta-propylene glycol; 1,3- propylene glycol; 1,3-Propanediol; | |
| 1,3-Dihydroxypropane; 1,3-Propandiol; Propane-1,3-diol; 2-Deoxyglycerol; 2-(Hydroxymethyl) ethanol; | ||
| DERIVATION | ||
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | clear liquid | |
| MELTING POINT | -27 C | |
| BOILING POINT | 210 C | |
| SPECIFIC GRAVITY | 1.05 - 1.06 | |
| SOLUBILITY IN WATER | Soluble | |
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
400 C | |
|
NFPA RATINGS |
Health: 1; Flammability: 2; Reactivity: 0 | |
|
REFRACTIVE INDEX |
1.438 - 1.440 | |
| FLASH POINT | 79 C | |
| STABILITY | Stable under ordinary conditions. Moisture sensitive | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Glycol: any of a class of organic chemicals characterized by having separate two
hydroxyl (-OH) groups, contribute to high water solubility, hygroscopicity and
reactivity with many organic compounds, on usually linear and aliphatic carbon
chain. The general formula is CnH2n(OH)2 or (CH2)n(OH)2. The broadened names
include diols, dihydric alcohols, and dihydroxy alcohols. Ethylene glycol,
HOCH2CH2OH, is the simplest member of the glycol family. Mono-, di- and
triethylene glycols are the first three members of a homologous series of
dihydroxy alcohols. Propylene glycol prepared by hydrolysis of propylene oxide
and widely used as an ingredient of antifreeze and humectant in cosmetics is
1,2-propanediol indicating the two hydroxyl group position at 1,2, while
trimethylene glycol is 1,3-propanediol with two hydroxyl group on the primary
carbon atoms. 1,3-propanediol is called beta-propylene
glycol. Trimethylene glycol is a clear, oily liquid; soluble in water;
soluble in oxygenated solvents and completely soluble
in alcohol; melting point -27 C; boiling point
210 C.
Trimethylene glycol has similar applications to Propylene glycol. It is can be used as a comonomer of unsaturated polyester resins, alkyd resins, polyester foams, polyester-based plasticizers, and as chain extender for polyurethane. It is a useful chemical intermediate which have two hydroxyl group on the primary carbon atoms and one alpha-carbon atom. 1,3-propanediol, or a derivative thereof, is used for the synthesis of lubricants, plasticizers, adhesives, photographic materials, pharmaceuticals, insect repellent, fragrances, antioxidant compound, antistatic agents, fabric softeners, and vitamin H. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear liquid | |
| ASSAY | 99.0% min | |
|
COLOR, APHA |
20 max |
|
| WATER |
0.2% max | |
| TRANSPORTATION | ||
| PACKING | 200kgs in drum | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: , Risk Phrases: , Safety Phrases: 23-24/25 | ||